A healthy boost to precision medicine in KSA
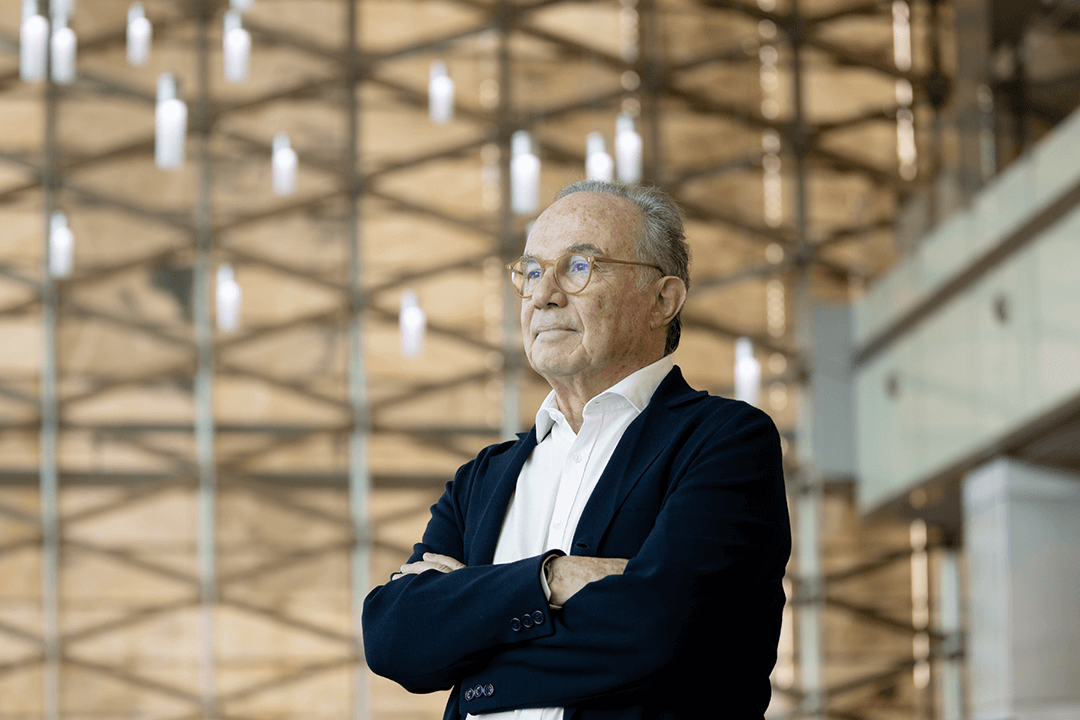
Professor Pierre Magistretti, director of the KAUST Smart Health Initiative, on the University's campus. Photo by Khulud Muath.
Originally published in Wired
About 15 years ago in a Kyoto lab, Japanese doctor Shinya Yamanaka rewrote the rules of genetics with the invention of a revolutionary technique to reprogram cells.
Yamanaka's breakthrough, for which he was later jointly awarded the Nobel Prize for medicine, showed how to change ordinary human cells into what he termed induced pluripotent stem (iPS) cells, which could then be reprogrammed to become any type of cell found in our bodies.
Previously that would have required embryonic stem cells, the use of which remains ethically controversial. Progress in developing iPS has been slow—it was not until 2017 that a Japanese man became the first person to receive cells from iPS provided by another person—but the potential is vast. Imagine a gene "bank" where patients could receive healthy, reprogrammed genes perfectly tailored to their own bodies in order to treat diseases and medical conditions.
That is the vision of Khaled Alsayegh, a Saudi molecular geneticist, whose stem cell research over the past decade has taken him from Virginia to Tokyo and now Jeddah, where he's associate director of biomedical research at the King Abdullah International Medical Research Center.
Alsayegh helped create the Saudi Stem Cell Donor Registry, which now comprises samples from 80,000 potential donors across the kingdom. His team is analyzing these to identify "universal" donors—people whose tissue type matches a large percentage of Saudi Arabia's population.
"When we've isolated these individuals, we'll collect their blood cells and reprogram these into iPS cells. These iPS cells will then be considered universal stock from which we can derive any tissue type," says Alsayegh. "These cells can be transplanted into anyone who can be matched using tissue typing."
While there are endless potential uses for these cells, Alsayegh is first focused on creating so-called pacemaker cells that could make the implanted devices obsolete. Electronic pacemakers typically need to be replaced every 5-15 years, forcing patients to undergo further surgery; the pacemaker cells would need only be transplanted once.
Crucially, by creating the gene bank, geneticists won't have to reprogram the patients' own cells, and can have suitable cells ready in advance.
"Whenever a patient has a need, we go into the bank and find a tissue type match and then we can immediately transplant it. That's the long-term goal," says Alsayegh.
To facilitate such complex work, Alsayegh has teamed up with Jesper Tegnér, a bioscience professor at KAUST, as part of the university's multimillion-dollar Smart Health Initiative, which launched in January. Tegnér will perform single-cell RNA sequencing to create the pacemaker cells.
"It's a very high-resolution technique in order to study the two cell types and compare them," says Alsayegh. "How do these cells we've produced in the lab compare with the real thing?"
Pre-clinical testing, using animals, could start within a year or two. Next would be human trials. "KAUST's facilities are amazing and collaborating with them is going to accelerate [the project]," adds Alsayegh.
"There's a gap between clinical care and research. What's missing is translational research, where scientists interact with clinicians."
The program is just one of 18 research projects to be awarded $100,000 seed grants as part of the Smart Health Initiative. For each, a KAUST scientist has partnered with a medical clinician in Saudi Arabia. Projects include studying gene mutations, identifying bacteria present in transplanted organs before transplantation, and understanding the pathway of leukemia.
They all represent what's known as translational research—"bench-to-bedside" programs that take biological knowledge and insights from clinical trials to treat particular medical conditions and diseases. Biologists and geneticists collaborate with experts in other fields including engineering, bioinformation, and pharmacy to enhance patient treatment.
"There's a gap between the excellence of clinical care and advanced clinical research… what's missing is translational research, where scientists interact with clinicians," says Pierre Magistretti, director of KAUST's Smart Health Initiative.
The scheme's partners include King Faisal Specialist Hospital, Johns Hopkins Aramco Healthcare and King Saud Medical City. The initiative will help train Saudi Arabia's next generation of clinician scientists and biomedical technologists, and includes an M.D.-Ph.D. program in which doctors will complete the research component of their doctorate at KAUST. The university hopes to show how combining clinical data with biomedical research and AI can accelerate advances in disease prevention, diagnosis, and treatment.
"First it's research, second education, and third is innovation and translation. The idea is that, from the Smart Health Initiative, there could be spinoffs from KAUST or the hospitals to create some value—companies that are active in biomedical, biopharma, biotechnology," says Magistretti.
KAUST will create a bio-innovation hub whereby startups or external companies can set up operations on campus, while the broader project's success will be measured over decades, says Magistretti. The first step, in terms of research, will be to identify the mechanisms of some genetic and metabolic diseases.
"If we would have some therapies for some of these diseases, that would be a great success," says Magistretti. "[We're] training a new generation of clinician scientists, MD-PhDs, and also technologists."
The Smart Health Initiative aims to enhance Saudi Arabia's capabilities in what Magistretti describes as precision medicine. He also highlights the kingdom's high prevalence of monogenic diseases, which are conditions caused by the modification of a single gene occurring in all cells of a human body. More than 10,000 diseases are known to be monogenic, according to the World Health Organization. Proteins encoded by particular genes are usually the cause of such diseases, says Magistretti.
"So, you have a hint, a clue about what physio-pathological mechanisms could be involved in the disease," he says. "Once you understand that, you can go for gene therapy, but [although] gene therapy is developing more and more, it's still not the panacea."
The initiative will profile the unique genetic variants in Saudi Arabia's population in order to better understand the disease-causing mechanisms. Of particular interest is diabetes. The prevalence of type 2 diabetes in Saudi Arabia is among the highest worldwide. "That's an interaction between vulnerability genes and lifestyle, so that will be one of the topics—metabolism, and the genetics of metabolism," says Magistretti.
Another of the initiative's projects is an analysis of the phenotypes of Saudi nationals. Phenotypes are an organism's observable traits that are a result of its genotype (set of heritable genes) interacting with its environment.
To conduct this study, Fowzan Alkuraya, professor of human genetics and principal clinical scientist at Riyadh's King Faisal Specialist Hospital and Research Centre, has teamed up with three KAUST scientists: Tegnér, computer science professor Xin Gao, and bioscience professor Stefan Arold.
"My lab has been on the hunt for naturally occurring human 'knockouts'—individuals who were essentially deprived of specific genes by virtue of damaging mutations to both copies of those genes," says Alkuraya.
Humans usually have two active copies of each gene, but a person can end up with both copies being inactive if their parents each have an active copy and an inactive copy due to a damaging mutation.
"Typically, these carriers do not suffer any major health consequences. However, their children are at risk of inheriting both inactive copies and this can have a wide range of effects on phenotypes," says Alkuraya. "This scenario is particularly common in Saudi Arabia due to the high rates of consanguinity." Or in other words, it's not unusual for Saudis to have parents who themselves are closely related.
A year-long pilot study will soon start, and will involve 25 staff. "This project will highlight novel therapeutic targets for a range of common disorders in the country. It will also provide valuable data for the pharmaceutical industry to inform their ongoing and future drug development efforts," says Alkuraya.