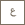KAUST team synthesizes novel metal-organic framework for efficient CO2 removal

Authors of a recent Nature Communications paper from Prof. Mohamed Eddaoudi's Functional Materials Design, Discovery & Development group (FMD3) work in the lab together to further their research on metal-organic frameworks (MOFs). From L-R: Drs. Youssef Belmabkhout, Karim Adil, Vincent Guillerm, and Osama Shekhah.
"In Professor Mohamed Eddaoudi's research group, we are always on the quest to find novel nanostructured functionalized materialsfor specific applications," explained KAUST Research Scientist Dr. Youssef Belmabkhout, a member of Prof. Eddaoudi's Functional Materials Design, Discovery, and Development (FMD3) group, part of KAUST's Advanced Membranes and Porous Materials (AMPM) research center.
Dr. Osama Shekhah, Senior Research Scientist in the FMD3 group added that the group searches "for materials that will be highly suitable for trace and low CO2 concentration removal using purely physical adsorption. These will help in energy saving and in the reduction of the cost of the production, purification, and enrichment of highly valuable commodities such as CH4, H2, O2, N2, and others."
Drs. Shekhah and Belmabkhout and a team of researchers from Prof. Eddaoudi's group recently discovered and synthesized a new porous, moisture-resistant, inexpensive and reusable copper-based metal-organic framework (MOF) called SIFSIX-3-Cu that can selectively adsorb and remove trace CO2 from mixtures of various gases. Their findings were published in the June 25 edition of Nature Communications (DOI: 10.1038/ncomms5228).
MOFs are a promising new class of hybrid solid-state materials for CO2 removal. "Their uniqueness," explained Prof. Eddaoudi, "resides in the ability to control their assembly and introduce functionality on demand. This feature is not readily available in other solid-state materials."
The researchers showed for the first time that MOF crystal chemistry permits the assembly of a new isostructural hexafluorosilicate MOF (SIFSIX-3-Cu) based on copper instead of zinc.
"This technology is anticipated to outperform the existing mature technologies for CO2 physical adsorption in terms of energy efficiency," says Dr. Shekhah. "The key factors for this finding are the combination of suitable pore size and high, uniform charge density in the pores of the MOF."
Using their newly synthesized MOF, the researchers examined the conditions relevant to direct air capture (DAC), a mechanism to remove CO2 from air and reduce greenhouse gas emissions uniformly around the world.
DAC is more challenging than post-combustion capture, but it may be practical if alternative "suitable adsorbent combining optimum uptake, kinetics, energetics and CO2 selectivity is available at trace CO2 concentration," the researchers stated.
The team discovered that contracting SIFSIX-3-Cu's pore system to 3.5 Å enhanced the material's efficiency, making it able to adsorb relatively large CO2 amounts 10-15 times higher than zinc-based metal-organic adsorbents, such as SIFSIX-3-Zn. In SIFSIX-3-Zn, the pore size is 3.84 Å.
"We attribute this property to enhanced physical sorption through the favorable electrostatic interactions between CO2 molecules and fluorine atoms present on the surface of the adsorbent," explained Zhijie Chen, a Ph.D. student in the FMD3 group and a co-author of the paper.
Dr. Vincent Guillerm, a post-doctoral fellow in the FMD3 group and a co-author of the paper also noted that, "the pore contraction gives CO2 uptake and selectivity at very low partial pressures. This is relevant to DAC and trace carbon dioxide removal."
"SIFSIX-3-Cu gives enhanced CO2 physical adsorption properties, uptake, and selectivity in highly diluted gas streams, and this performance is unachievable with other classes of porous materials," added Dr. Karim Adil, a co-author of the paper and Research Scientist in the FMD3 group.
The researchers are excited about their finding as it offers the potential to be used not only for DAC but also for other applications related to energy, the environment, and the healthcare field. For example, SIFSIX-3-Cu could be used to remove and recycle CO2 in confined spaces, such as in submarines or space shuttles, and could also be used in anesthesia machines, which require efficient CO2 sorbents.
"Our work paves the way for scientists to develop new separation agents suitable for challenging endeavor pertaining to CO2 ultra-purification processing," said Dr. Shekhah. "Our study is also part of a greater critical effort to develop economical and practical pathways to reduce cumulative CO2 emissions provoking the undesirable greenhouse gas effect."
Prof. Eddaoudi reiterated that "MOFs offer remarkable CO2 physical adsorption attributes in highly diluted gas streams thanks to their ability for rational pore size modification and inorganic-organics moieties substitution. Other classes of plain materials are unable to attain this."
In the future, Prof. Eddaoudi's FMD3 group will continue to develop topologically and chemically different MOFs. "We aim to target novel MOFs with suitable pore size and high charge density," explained Prof. Eddaoudi. "We will then use these for the important task of removing trace and low and high concentration CO2."
By Caitlin Clark, KAUST News