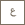Researchers create salts for cheap and efficient CO2 capture

Dr. Thien Nguyen (left) and Professor Cafer T. Yavuz (right) pose with a bottle of CO2-loaded guanidinium sulfate salt (held by Dr. Nguyen). The ten gas-filled, two-liter bottles in front represent the required space for the same amount of carbon dioxide that would be needed were it not being held in clathrate form (small bottle). Photo: KAUST
A team of international researchers led by Professor Cafer T. Yavuz of King Abdullah University of Science and Technology (KAUST), Prof. Bo Liu from University of Science and Technology of China (USTC), and Prof. Qiang Xu of Southern University of Science and Technology (SUSTech) have developed a promising method for carbon capture and storage.
Methane hydrate is studied for its ability to capture and trap gas molecules such as carbon dioxide under high pressure. However, it is difficult to impossible to recreate these conditions in the lab, and the approach is additionally energy intensive, as the methane-ice solid requires refrigeration. Using a salt — guanidinium sulfate — the scientists have successfully created lattice-like structures called clathrates that effectively mimic the methane hydrate activity, trapping the CO2 molecules and resulting in an energy efficient way to contain the greenhouse gas.
“The guanidinium sulfateserves to organize and trap the CO2 molecules without reacting with them,” said Cafer Yavuz, professor of chemistry, and director of the KAUST Oxide and Organic Nanomaterials for Energy & Environment (ONE) Laboratory.“We have discovered a rare example of a clathrate that is stable and non-corrosive at ambient temperature and pressure, a highly desirable feature compared with ethanol amine, ammonia and other solutions that are commonly used in carbon capture.”
Previous carbon capture methods included chemisorption, where chemical bonds form between CO2 molecules and the surface. This process worked well; however, the chemical bonds require energy to break them down, which drives up the cost of the CO2 capture operation. The salt-based, clathrate structure utilizes low energy, physisorption processes while capturing CO2 without water or nitrogen interference, opening a promising venue for future carbon capture and storage technologies through rapid CO2 solidification.
The discovery introduces a new way of storing and transporting carbon dioxide as a solid. CO2 is conventionally carried as a solid in the form of dry ice; compressed in gas cylinders; or in the form of carbonates. The salt clathrate allows CO2 to be carried as a solid powder, yielding remarkably high volume per weight capacity, making this method the least energy intensive, with tremendous potential for real life applications.
"Our team made it possible to carry CO2 in a solidform without the need for refrigeration or pressure. You will be able to literally shovel CO2 loaded solids from now on,” he said. “The impact is wide and strong, as the global fuel industry and the Kingdom entities are actively looking for ways to capture, store and transport CO2 without significant energy penalties."
The breakthrough could have a significant impact on the fight against climate change, enabling energy-efficient carbon capture and storage. The research team is optimistic that their findings will lead to further improvements in CO2 capture in terms of stability, recyclability, sorption capacity and selectivity, and lowering regeneration energy penalty and cost.
The research was carried out at Southern University of Science and Technology, University of Science and Technology of China, and King Abdullah University of Science and Technology. The research findings have been published in the journal Cell Reports Physical Science.